Biosimilars: What They Are, How They Compare to Brand-Name Drugs, and Why They Matter
When you hear biosimilars, copycat versions of complex biologic drugs that are made from living cells, not chemicals. Also known as biologic generics, they offer the same clinical benefits as the original drug—but at a fraction of the cost. Unlike regular generics, which are simple chemical copies, biosimilars are made from living organisms like bacteria or yeast, making them far more complex to produce. That’s why they’re not exact copies, but highly similar—so similar that the FDA requires them to show no meaningful difference in safety or effectiveness.
These drugs are used for serious conditions like rheumatoid arthritis, Crohn’s disease, cancer, and diabetes. For example, adalimumab, a biologic used to treat autoimmune diseases has multiple biosimilars now on the market, cutting prices by up to 60%. The same goes for insulin, a life-saving biologic for diabetics, where biosimilars are finally making treatment more affordable after decades of sky-high prices. Even though they’re biologically complex, regulators like the FDA and EMA demand strict testing—especially for narrow therapeutic index drugs, medications where tiny dosage changes can cause serious harm. That’s why biosimilars go through more testing than regular generics.
Many people worry biosimilars aren’t as safe, but studies show they work just as well. In fact, millions of patients in Europe and the U.S. have used them for over a decade with no increase in side effects. The real difference? Cost. A single dose of a brand-name biologic can cost $2,000 or more. A biosimilar? Often under $800. That’s why Medicaid and private insurers are pushing them harder in 2025—especially for long-term treatments. You’ll find posts here that break down how biosimilars fit into drug coverage rules, how they’re tested for safety, and how they stack up against the originals. Whether you’re on a biologic now or considering switching, this collection gives you the facts without the hype.
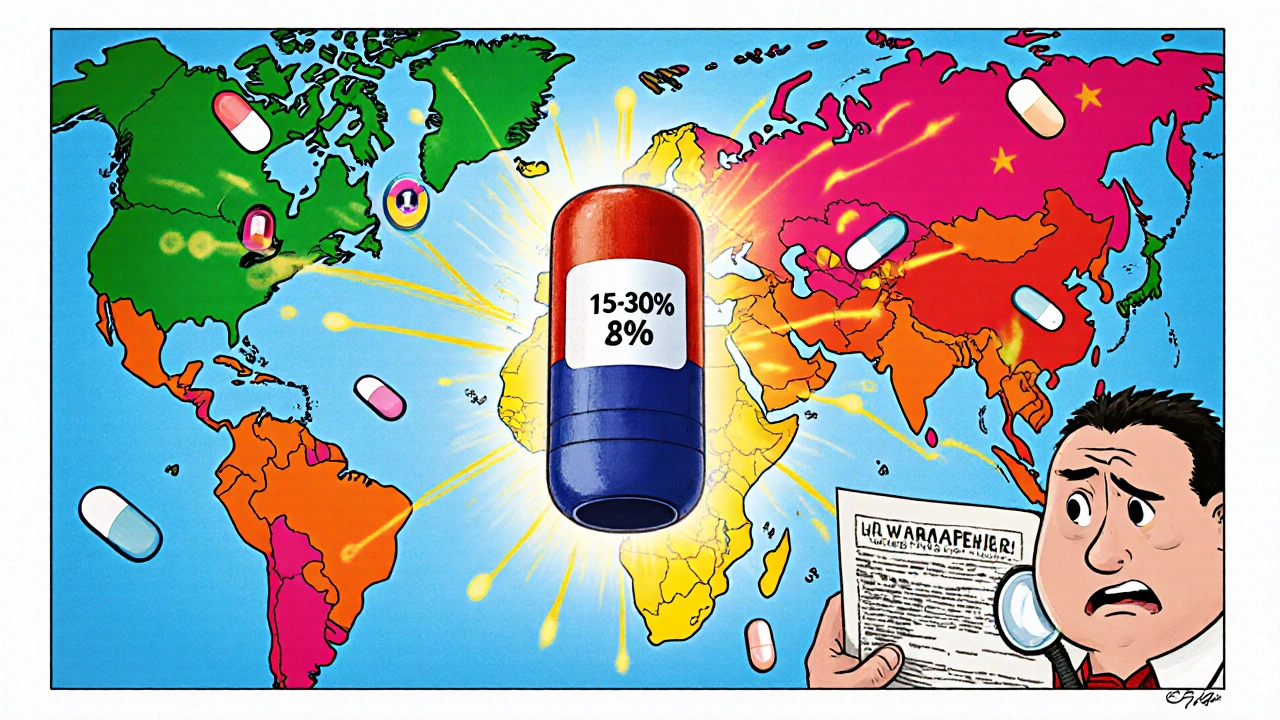
Future of Global Generic Markets: Key Predictions and Trends Through 2030
The global generic drugs market is growing rapidly, driven by cost pressures and chronic disease trends. Key trends include the rise of biosimilars, expansion in pharmerging markets, supply chain risks, and tightening regulations through 2030.
View More