Pharmerging Markets: What They Are and Why They Matter for Medications
When we talk about pharmerging markets, fast-growing regions where pharmaceutical demand is rising rapidly due to population growth, urbanization, and improving healthcare access. Also known as emerging pharmaceutical markets, these areas include countries like India, Brazil, Nigeria, and Indonesia—places where millions are gaining access to prescription drugs for the first time. This isn’t just about more pills being sold. It’s about whether those pills are safe, affordable, and actually reach the people who need them.
These markets rely heavily on generic drugs, lower-cost versions of brand-name medications that meet the same safety and effectiveness standards. Also known as off-patent medicines, generics make up over 90% of prescriptions in places like the U.S., and even higher percentages in pharmerging regions where price is the biggest barrier. But here’s the catch: not all generics are created equal. Some come from factories with weak oversight, leading to dangerous variations in quality. That’s why regulators like the FDA and EMA have strict rules for bioequivalence, the measure that proves a generic drug performs the same way in the body as the original. Also known as therapeutic equivalence, this standard keeps patients safe when switching from brand to generic. For drugs with a narrow therapeutic index—like blood thinners or seizure meds—this isn’t just important, it’s life-or-death.
Pharmerging markets also force a rethink of drug pricing, how much medicines cost and who pays for them. Also known as pharmaceutical affordability, this issue drives everything from Medicaid formularies in the U.S. to government bulk-buying programs in Africa. In places with no universal healthcare, people choose between medicine and food. That’s why programs like India’s public drug distribution or Brazil’s Farmácia Popular exist—to bring down costs and stretch limited budgets. But they can’t work without reliable supply chains, trained pharmacists, and clear labeling. That’s where health literacy comes in. If patients don’t understand their prescriptions, even the cheapest drug won’t help.
What you’ll find in this collection are real-world examples of how these forces play out: how a generic version of a blood thinner must meet exact standards to be approved, how Medicaid in the U.S. decides which drugs to cover, and how people in low-income countries access life-saving meds through local clinics and community pharmacies. You’ll see how regulations like the FD&C Act and Hatch-Waxman Amendments shaped today’s generic drug system—and why those same rules are now being adapted overseas. There’s no fluff here. Just clear, practical insights into how medicines get made, priced, and delivered in the parts of the world where need is greatest and resources are tight.
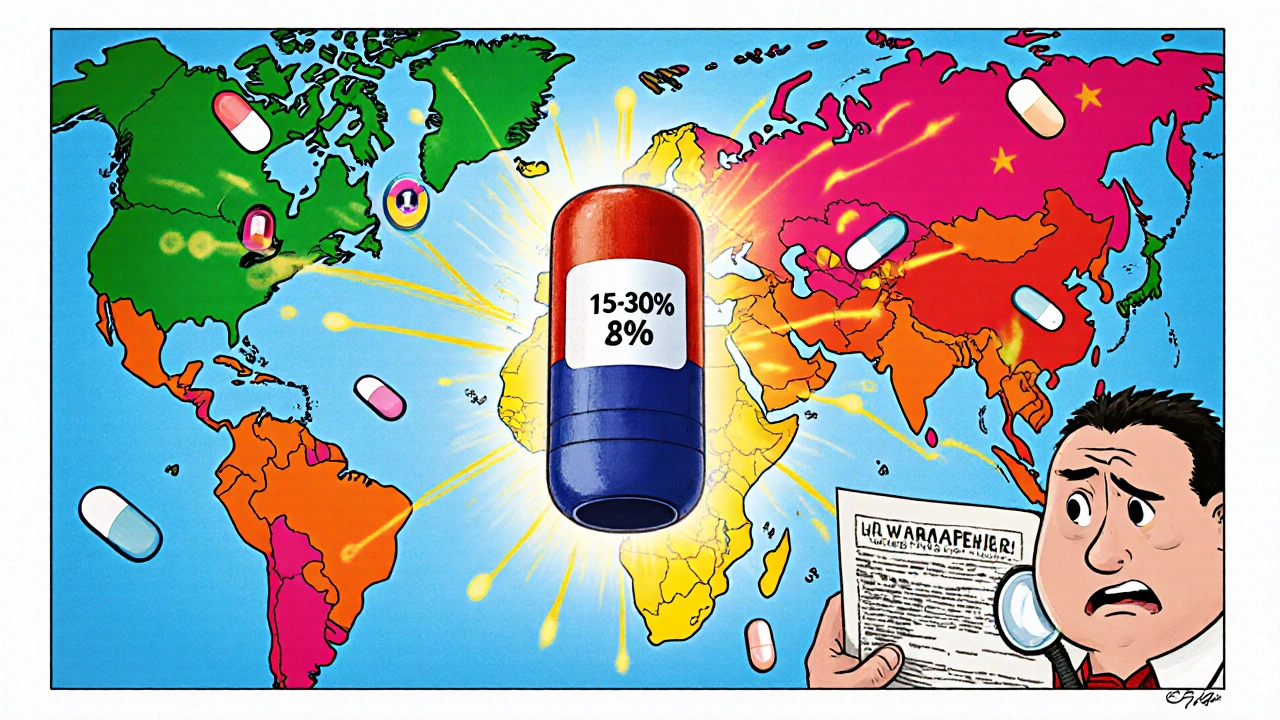
Future of Global Generic Markets: Key Predictions and Trends Through 2030
The global generic drugs market is growing rapidly, driven by cost pressures and chronic disease trends. Key trends include the rise of biosimilars, expansion in pharmerging markets, supply chain risks, and tightening regulations through 2030.
View More