When you pick up a prescription, you might see two options: the familiar brand-name pill or a much cheaper generic version. Many people wonder-does the generic really work the same? Is it just a cheaper copy, or is it truly equivalent? The answer isn’t just marketing-it’s backed by decades of clinical research, regulatory science, and real-world patient data.
What Makes a Generic Drug "Equal"?
A generic drug isn’t just a lookalike. It must contain the exact same active ingredient, in the same strength, and delivered the same way as the brand-name version. That means if you’re taking 10 mg of lisinopril for high blood pressure, the generic version has the same 10 mg of lisinopril, same tablet form, same way it’s absorbed. The FDA requires this before approval.
But here’s the key: it’s not enough to have the same ingredient. The body has to absorb it the same way. That’s where bioequivalence comes in. The FDA requires generic manufacturers to prove their product is absorbed into the bloodstream at the same rate and to the same extent as the brand-name drug. The standard? The amount of drug in the blood (measured as AUC and Cmax) must fall within 80% to 125% of the brand-name drug’s levels. This isn’t a guess-it’s a strict statistical test based on studies with 24 to 36 healthy volunteers.
Since 1984, when the Hatch-Waxman Act created the modern generic approval system, over 90% of all prescriptions filled in the U.S. have been generics. And yet, they make up only about 23% of total drug spending. That’s because generics cost, on average, 80% to 85% less than brand-name drugs. That’s not a small savings-it’s life-changing for people on fixed incomes, especially those managing chronic conditions like diabetes, hypertension, or depression.
What Do Large-Scale Studies Say?
One of the most comprehensive studies came from Austria in 2020, published in Scientific Reports. Researchers analyzed data from nearly every insured person in the country between 2007 and 2012, comparing outcomes for 17 different drugs-everything from statins to antidepressants. The results were striking. For 10 out of the 17 drugs, patients taking generics had fewer deaths. For 11 out of 17, they had fewer major heart events. After adjusting for age, income, and other health factors, the five-year survival rate for those on generics was 82.7%, compared to 79.8% for those on brand-name drugs. That’s not a small difference. It’s a real benefit.
Another massive analysis in 2019, led by Dr. R.J. Desai and published in PLoS ONE, looked at 3.5 million patients across two U.S. databases. They studied common drugs like amlodipine (for blood pressure), glipizide (for diabetes), and escitalopram (for depression). The results? No meaningful difference in clinical outcomes. In fact, for amlodipine and its combination with benazepril, patients on generics had slightly lower risk of heart attacks and hospitalizations. The hazard ratios were 0.91 and 0.84-meaning generics were associated with fewer bad outcomes.
Even for heart failure and hypertension, a 2022 study in JAMA Internal Medicine followed 1.2 million patients on ACE inhibitors. They found no difference in hospitalization rates between brand-name and generic versions. The heart doesn’t care if the pill is called Lisinopril or just "lisinopril"-it responds to the same molecule.
Where Do Things Get Tricky?
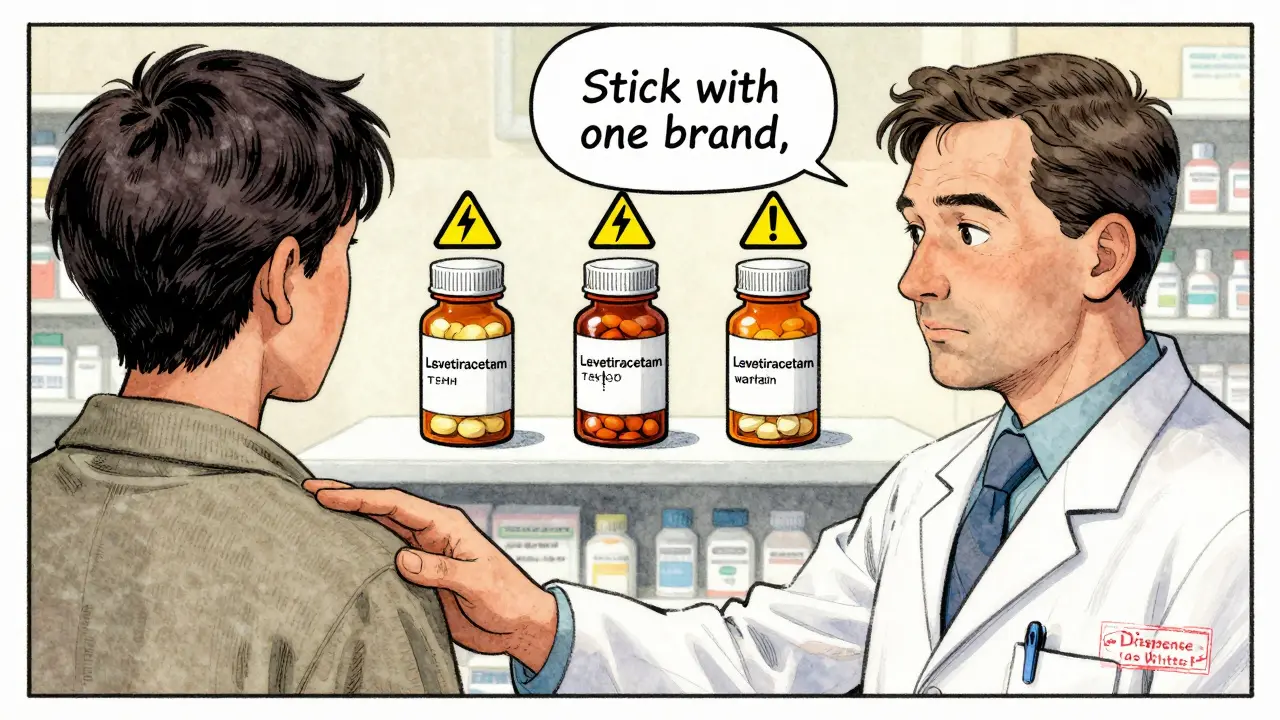
Not all drugs are created equal in how they’re absorbed. Some have what’s called a narrow therapeutic index. That means the difference between a dose that works and one that’s toxic-or ineffective-is tiny. For these drugs, even a small change in how the body absorbs the medicine can matter.
Drugs like levothyroxine (for thyroid), warfarin (for blood thinning), and certain anti-seizure medications fall into this category. That’s why some neurologists report breakthrough seizures when patients switch between different generic versions of lamotrigine or levetiracetam-even though each version meets FDA bioequivalence standards. A 2023 study in Epilepsia found a 18% higher chance of seizure recurrence after switching between generic versions of levetiracetam.
Why? Because even though the active ingredient is the same, the fillers, coating, or manufacturing process can affect how quickly the pill breaks down in the stomach. For most drugs, that doesn’t matter. For these, it can. That’s why doctors sometimes write "dispense as written" on prescriptions for these drugs. It’s not because generics are bad-it’s because the margin for error is razor-thin.
And then there’s the case of levothyroxine. Thousands of patients report that one generic brand works perfectly, but another causes fatigue, weight gain, or heart palpitations. Why? Because thyroid hormone levels are incredibly sensitive. A 2023 survey by GoodRx showed that while 78% of users said generics worked just as well as brand-name Synthroid, a vocal minority reported clear differences-especially when switching between manufacturers. The FDA acknowledges this and recommends staying with the same generic brand if you’ve found one that works.

Are There Real Cases Where Generics Failed?
Yes-but they’re rare, and often misunderstood. A 2013 study by Dr. L. Gallelli reviewed over 2,000 bioequivalence studies and found no overall difference in effectiveness. But it also documented individual cases where patients had problems after switching. One patient on generic levofloxacin (Ranbaxy) for a lung infection kept getting worse. After switching back to the brand-name Tavanic, symptoms cleared within 10 days. Another patient with chronic pain had no relief on generic oxycodone but responded immediately to the brand.
These aren’t proof that generics are inferior. They’re proof that human biology is complex. Sometimes, it’s not the drug-it’s the patient’s body reacting to a change in inactive ingredients. A different dye, binder, or coating might cause a mild allergic reaction or alter gut absorption just enough to make a difference in someone who’s already fragile.
It’s also worth noting that about 30% of patients in some studies reported no change, 30% felt better, 10% had side effects, and 30% stopped taking the drug altogether. That last group often didn’t realize their symptoms were due to the switch-not the disease getting worse.
What Do Experts Really Think?
Most experts agree: generics work. Dr. Niteesh Choudhry from Harvard Health said, "The existing data are reassuring." The FDA’s Dr. Sarah Ibrahim has repeatedly stated that generics have the same active ingredients, safety, and effectiveness as brand-name drugs. The American Family Physician journal confirmed in 2018 that multiple studies show generics are equally effective.
But even experts don’t ignore the exceptions. A 2017 Canadian study in Circulation: Cardiovascular Quality and Outcomes found higher rates of side effects after switching elderly patients to generic versions of blood pressure drugs like losartan and valsartan. The researchers didn’t say generics were unsafe-they said the change itself might trigger reactions in older, multi-medication users.
The bottom line? Generics are safe and effective for the vast majority of people. But for certain high-risk drugs, and for patients who’ve been stable on one brand, switching without medical guidance can be risky.
What About Patient Experiences?
Real-world feedback tells a mixed but mostly positive story. Medicare beneficiaries surveyed in 2021 said 68% noticed no difference in effectiveness when switching to generics. Another 22% had minor side effects that went away after a few weeks. Only 10% reported persistent problems.
On platforms like Reddit and pharmacy forums, patients are candid. One user wrote: "I’ve tried three different generics for Synthroid. Only one kept my TSH stable." Another said: "I switched to generic Keppra and had a seizure I hadn’t had in two years. Went back to brand-never had another." These aren’t anecdotes to dismiss-they’re signals that for some, consistency matters.
Meanwhile, GoodRx’s 12,450 reviews show a 4.2 out of 5-star rating for generics, with 78% saying they work just as well as brand-name drugs at a fraction of the cost. That’s not luck. That’s science working as intended.
What Should You Do?
If you’re taking a generic drug and feel fine-keep taking it. There’s no reason to switch unless your doctor suggests it.
If you’re switching from brand to generic and notice changes-fatigue, mood shifts, new side effects, or worsening symptoms-don’t assume it’s "all in your head." Talk to your doctor. Keep a symptom log. Your pharmacist can help you identify which generic manufacturer you’re getting and whether switching back might help.
If you’re on a drug with a narrow therapeutic index-like levothyroxine, warfarin, or an anti-seizure medication-ask your doctor if you should stick with one brand or generic. Some insurance plans force switches; you have the right to request an exception if it affects your health.
And if cost is your biggest concern? Generics are the smartest choice. The FDA, CDC, and Medicare all agree: they save billions every year without sacrificing safety. For 90% of people, they’re just as good-and sometimes better.
What’s Changing Now?
The FDA is tightening its rules for complex generics-like inhalers, injectables, and topical creams-because these are harder to replicate. Starting in 2025, some of these will require additional human studies, not just lab tests. That’s a good thing. It means more accurate equivalence testing.
Manufacturing quality is also under scrutiny. In 2021, a global recall of valsartan (a blood pressure drug) affected multiple generic brands because of a cancer-causing impurity. That wasn’t about efficacy-it was about contamination. The FDA now inspects more foreign manufacturing plants and requires stricter controls.
The bottom line? Generics are not a compromise. They’re a triumph of science and policy. They make life-saving medicine affordable. The research is clear: for most people, most of the time, they work just as well. The exceptions? They exist-but they’re the rule, not the norm.
15 Comments
Siobhan Goggin
January 4, 2026 at 18:58 PM
The data is overwhelming. Generics aren’t just cheaper-they’re clinically equivalent for the vast majority of patients. It’s irresponsible to spread fear without evidence.
Jay Tejada
January 5, 2026 at 05:10 AM
yeah right. i switched my mom from Synthroid to generic and she started nodding off at the dinner table. now she’s back on brand. not every body’s a lab rat.
Allen Ye
January 5, 2026 at 12:24 PM
Let’s be honest-this whole debate is less about pharmacology and more about trust. We’ve been conditioned to equate price with value, and brand names with quality. But the science doesn’t care about logos. The molecule doesn’t know if it was made in New Jersey or Hyderabad. What matters is bioavailability, not branding. And yet, we treat generics like they’re the cheap knockoff version of a Rolex, when in reality, they’re the same movement in a different case. The FDA doesn’t approve generics because they’re ‘good enough’-they approve them because they’re statistically identical in absorption, dissolution, and effect. The real tragedy isn’t that people doubt generics-it’s that we still let corporate marketing dictate our health choices.
mark etang
January 6, 2026 at 14:35 PM
It is imperative that patients understand the rigorous regulatory standards governing generic pharmaceuticals. The Food and Drug Administration mandates bioequivalence testing with stringent statistical thresholds. To suggest otherwise is to undermine public health infrastructure.
josh plum
January 8, 2026 at 04:43 AM
oh sure, the FDA says it’s fine. but did you know the same companies that make brand-name drugs also make the generics? they just slap a new label on it. it’s all a scam to make you think you’re saving money while they just pocket the difference. and don’t even get me started on the Indian factories-half of them are barely regulated. you think your generic levothyroxine is safe? think again.
John Ross
January 9, 2026 at 18:04 PM
Pharmacokinetic variability in narrow therapeutic index drugs remains a clinically significant concern. While AUC and Cmax fall within 80–125% per FDA guidelines, inter-individual absorption variance-particularly in polymorphic CYP450 metabolizers-can yield subtherapeutic or toxic exposure profiles. This is not theoretical; it’s documented in case series for lamotrigine and warfarin. Generic substitution without therapeutic monitoring constitutes a risk stratification failure.
jigisha Patel
January 10, 2026 at 11:43 AM
Let’s analyze this objectively. The Austrian study you cited had a 2.9% survival advantage for generics-but it failed to control for medication adherence rates. Patients on generics are more likely to refill prescriptions due to cost savings, which confounds the outcome. Also, the PLoS ONE study used retrospective data with potential selection bias. The real issue isn’t efficacy-it’s the lack of longitudinal, double-blind trials comparing generics across diverse populations.
Justin Lowans
January 11, 2026 at 01:31 AM
Man, I used to think generics were sketchy too-until I started working in a pharmacy and saw how many people were skipping doses because they couldn’t afford the brand. One guy told me he’d been rationing his insulin because the brand cost $400. Switched him to generic-same A1c, same energy, same life. That’s not magic. That’s justice.
Michael Rudge
January 11, 2026 at 05:33 AM
Wow. So you’re telling me I should trust a pill made in a factory that got shut down last year for falsifying lab data? Yeah, no thanks. I’ll pay extra to not die because some CEO decided to cut corners.
Doreen Pachificus
January 12, 2026 at 23:14 PM
my doc switched me to generic metoprolol and I felt like a zombie for two weeks. then I switched back and boom-back to normal. i’m not saying generics don’t work, i’m saying my body doesn’t like the filler in that one brand.
Cassie Tynan
January 14, 2026 at 14:56 PM
so the FDA says it’s fine, but when you switch your thyroid med and suddenly you’re crying over a commercial for dog food… maybe the ‘same molecule’ thing isn’t the whole story? just saying.
Jack Wernet
January 15, 2026 at 23:21 PM
The ethical imperative to ensure access to affordable medication must be balanced with the duty to preserve therapeutic stability. For patients with chronic, life-sustaining conditions, consistency in formulation is not a luxury-it is a clinical necessity.
Charlotte N
January 17, 2026 at 16:16 PM
i switched to generic celexa and my anxiety got worse… but then i switched back and felt better… so i guess it’s the stuff inside the pill? idk. i just know i don’t wanna risk it anymore
Jacob Milano
January 17, 2026 at 22:49 PM
my grandma’s on generic warfarin. her INR’s been stable for 3 years. she’s 84, lives on Social Security, and she’s alive because generics exist. the people who say they don’t work? they’ve never had to choose between rent and medicine. so yeah, i’ll take my 80% cheaper pill and thank the scientists who made it possible.




Peyton Feuer
January 4, 2026 at 07:03 AM
i used to swear by brand-name meds until my insurance forced me to switch to generic lisinopril. no difference in my blood pressure, and i saved $80 a month. honestly? i’m shocked more people don’t get this.