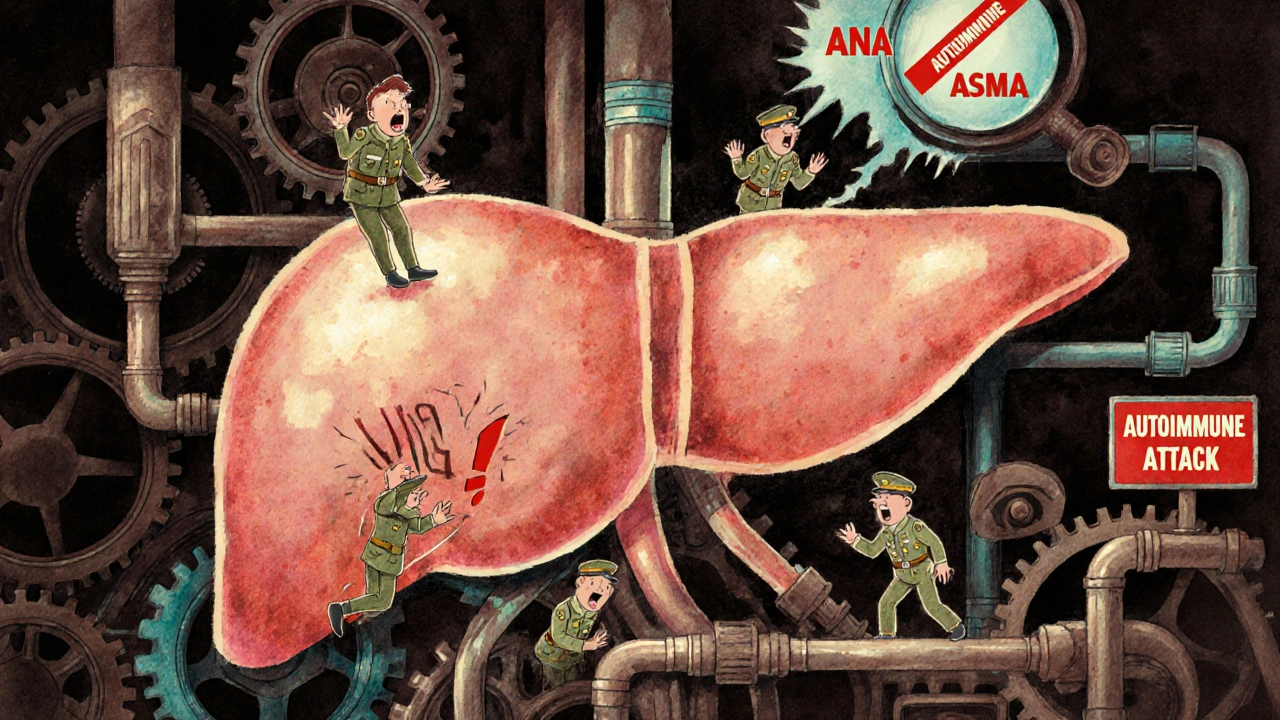
Autoimmune hepatitis is not caused by alcohol, viruses, or fatty foods. It happens when your immune system - the very thing meant to protect you - turns against your liver. Instead of fighting off germs, it starts attacking healthy liver cells. This triggers chronic inflammation, which over time can scar the liver, lead to cirrhosis, or even liver failure. Unlike viral hepatitis, you can’t catch it from someone else. It’s not caused by lifestyle. It’s an internal mistake - your body’s own defenses go rogue.
How Autoimmune Hepatitis Works
Your liver is a silent powerhouse. It filters toxins, makes proteins, stores energy, and helps digest food. When autoimmune hepatitis strikes, your immune system sees liver cells as foreign invaders. It sends in white blood cells and antibodies to destroy them. The result? Inflammation. That inflammation doesn’t go away. It keeps going, slowly chewing through healthy tissue.
This isn’t sudden. Most people don’t wake up one day with jaundice. Symptoms creep in: extreme fatigue, joint pain, nausea, dark urine, or a vague feeling of being unwell. Some don’t feel anything at all - the disease is found only during routine blood tests showing elevated liver enzymes. About 25% of cases appear suddenly, mimicking acute viral hepatitis, which often leads to misdiagnosis.
There are two main types. Type 1 is the most common - making up 80-90% of cases in the U.S. and Europe. It often shows up in teens and young adults, and more than three times as many women as men get it. Type 2 is rarer and mostly affects children between ages 2 and 14. The difference isn’t just age - it’s the antibodies your body produces. Type 1 shows antinuclear antibodies (ANA) or anti-smooth muscle antibodies (ASMA). Type 2 shows anti-LKM-1 or anti-LC-1 antibodies. These aren’t just labels. They help doctors confirm the diagnosis.
Diagnosing the Invisible Enemy
There’s no single test for autoimmune hepatitis. Diagnosis is a puzzle. Doctors piece together blood work, antibody tests, liver biopsy results, and your medical history. First, they rule out other causes: hepatitis B or C, alcohol use, fatty liver disease, or drug reactions. About 15-20% of autoimmune hepatitis cases are initially mistaken for drug-induced liver injury because the symptoms overlap so closely.
Blood tests are the first clue. Elevated ALT and AST - liver enzymes - are usually 5 to 10 times higher than normal. IgG levels, a type of antibody, are often more than 1.5 times above normal. That’s called hypergammaglobulinemia. It’s a sign your immune system is overactive. Bilirubin may rise too, especially if the disease is advanced.
The gold standard is a liver biopsy. A tiny sample of liver tissue is examined under a microscope. Pathologists look for three key signs: interface hepatitis (where immune cells invade the border between liver tissue and blood vessels), lymphoplasmacytic infiltration (clusters of immune cells), and rosette formation (hepatocytes arranged in rings). Fibrosis is scored using the METAVIR system - from F0 (no scarring) to F4 (cirrhosis). This tells doctors how far the damage has gone.
The Revised International Autoimmune Hepatitis Group Scoring System, updated in 2022, combines all these factors into a point system. It’s 92% sensitive and 97% specific when used by experienced hepatologists. That means it’s extremely accurate. A newer biomarker, anti-SLA/LP antibodies, has been added to the criteria. If present, it boosts diagnostic accuracy to 99%.
Standard Treatment: Immunosuppression, Not Antivirals
Since your immune system is the problem, you don’t need antiviral drugs. You need to calm it down. The first-line treatment is a combination of prednisone (a corticosteroid) and azathioprine (an immunosuppressant). Prednisone reduces inflammation quickly. Azathioprine helps maintain long-term control and lets doctors lower the steroid dose over time.
Typical starting doses: prednisone at 0.5 to 1 mg per kg of body weight (up to 60 mg daily) and azathioprine at 50 mg daily. Within 3 to 6 months, most patients see their liver enzymes drop. Remission - defined as normal ALT, AST, and IgG levels for at least two years - is achieved in 65-80% of patients, according to long-term data from the International Autoimmune Hepatitis Group.
But steroids aren’t harmless. Side effects are real and often harsh: weight gain, mood swings, insomnia, increased risk of infections, bone thinning, and diabetes. That’s why doctors aim to taper prednisone down to the lowest possible dose - ideally off it entirely - while keeping azathioprine going. About 25% of patients need low-dose maintenance therapy for life.
For those who can’t tolerate azathioprine - about 15-20% - mycophenolate mofetil is the next option. Studies show it works in 70-80% of these patients. It’s not FDA-approved specifically for autoimmune hepatitis, but it’s widely used and supported by major liver centers like NewYork-Presbyterian.
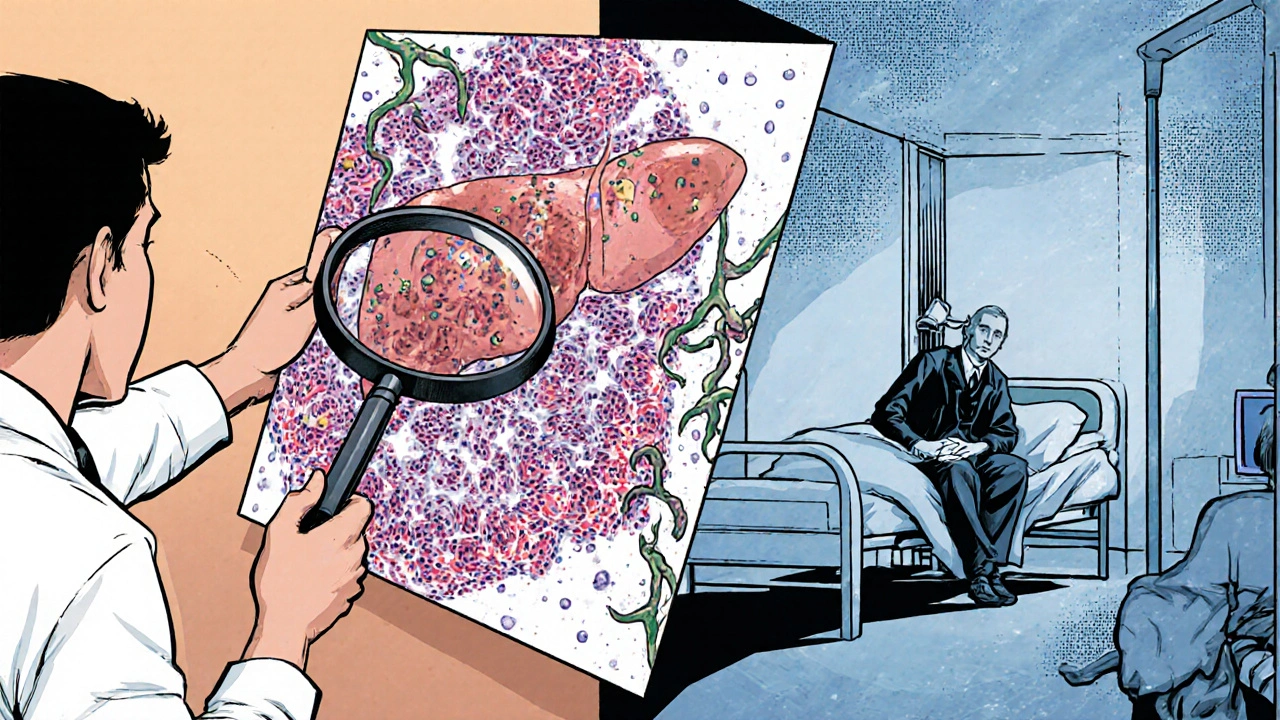
What Happens If You Don’t Treat It
Without treatment, autoimmune hepatitis is deadly. Ten-year survival drops to just 10%. With treatment, it jumps to 94%. That’s not a small difference - it’s the difference between life and death.
Left unchecked, inflammation leads to fibrosis, then cirrhosis. Once cirrhosis sets in, the liver can’t regenerate properly. Complications like fluid buildup in the abdomen, bleeding from swollen veins in the esophagus, or confusion from toxin buildup (hepatic encephalopathy) become likely. At that stage, a liver transplant may be the only option.
Even with treatment, about 10% of patients don’t respond well. These are the treatment-refractory cases. They keep progressing despite optimal therapy. For them, liver transplantation is often the next step. Autoimmune hepatitis is the fourth leading reason for adult liver transplants in the U.S., accounting for 6.2% of all transplants in 2022.
Living With Autoimmune Hepatitis
Managing this disease isn’t just about pills. It’s about lifestyle, monitoring, and mental health.
Regular blood tests are non-negotiable. During the first few months of treatment, you’ll need them every 2-4 weeks. Once stable, every 3 months is typical. Monitoring IgG and liver enzymes tells your doctor if the treatment is working or if you’re relapsing.
Bone health is a hidden risk. Steroids weaken bones. The National Osteoporosis Foundation recommends daily calcium (1,200 mg) and vitamin D (800-1,000 IU) for all patients on long-term prednisone. Weight-bearing exercise helps too.
Fatigue is the most common complaint. In a 2022 survey of over 1,000 patients, 78% said fatigue was their worst symptom. Joint pain affected 63%. Many report feeling like they’re constantly fighting just to get through the day. Mental health suffers too. Seventy-one percent said they felt anxious about disease progression. Fifty-four percent said their symptoms made it hard to keep a job.
Some patients find relief. One woman on CaringBridge wrote that her ALT dropped from 480 to 32 in six weeks after starting treatment. Others struggle with the emotional toll. One Reddit user described feeling like a “prisoner to medication” after seven years of treatment, constantly worried about infections or side effects.

The Future: What’s Next for AIH Treatment
There’s no new FDA-approved drug for autoimmune hepatitis since 2002. But the pipeline is active. Clinical trials are testing biologics like rituximab (which targets B-cells) and vedolizumab (which blocks immune cell migration to the gut and liver). Early results are promising.
In 2022, the European Medicines Agency gave orphan drug status to obeticholic acid after a phase 2 trial showed 45% of patients reached complete remission in 24 weeks - nearly double the placebo rate. It’s not yet approved, but it’s a sign that targeted therapies are coming.
The biggest shift on the horizon is personalized medicine. Researchers have found strong links between autoimmune hepatitis and specific genetic markers: HLA-DRB1*03:01 and HLA-DRB1*04:01. Patients with these alleles respond differently to treatment. Some respond better to azathioprine. Others do better with mycophenolate. Within the next 5-7 years, genetic testing could guide therapy choices, boosting remission rates to 85-90% while cutting side effects.
The global treatment market for autoimmune hepatitis is growing fast - projected to hit $2.04 billion by 2028. That growth isn’t just about more drugs. It’s about better diagnosis, earlier detection, and smarter management. More people are being identified now than ever before. That’s good news - because the earlier you treat it, the better your chances.
Key Takeaways
- Autoimmune hepatitis is a chronic condition where the immune system attacks the liver - not caused by viruses or lifestyle.
- Two main types: Type 1 (most common, affects adults, ANA/ASMA positive) and Type 2 (affects children, LKM-1/LC-1 positive).
- Diagnosis requires blood tests, antibody screening, and often a liver biopsy using the METAVIR scoring system.
- First-line treatment: prednisone + azathioprine. Remission rates are 65-80% with proper therapy.
- Without treatment, 90% of patients die within 10 years. With treatment, survival jumps to 94%.
- Steroid side effects are common - weight gain, bone loss, mood changes - so monitoring and bone health support are essential.
- Up to 10% of patients don’t respond to standard treatment and may need a liver transplant.
- Future treatments include biologics and genetic-guided therapy, which could make treatment more effective and less toxic.
Can autoimmune hepatitis be cured?
Autoimmune hepatitis can’t be completely cured, but it can be effectively controlled. Most patients achieve long-term remission with medication. Remission means liver enzymes and IgG levels are normal, inflammation is gone, and the disease isn’t progressing. Many people live normal, healthy lives for decades. But stopping treatment usually leads to relapse, so most need ongoing monitoring and often low-dose medication for life.
Is autoimmune hepatitis hereditary?
It’s not directly inherited like a genetic disease, but your genes play a big role. Certain gene variants - especially HLA-DRB1*03:01 and HLA-DRB1*04:01 - significantly increase risk. If a close family member has autoimmune hepatitis or another autoimmune disease (like type 1 diabetes or rheumatoid arthritis), your risk is higher. But having the gene doesn’t mean you’ll get it. Environmental triggers - like infections or medications - are likely needed to start the immune attack.
Can you drink alcohol with autoimmune hepatitis?
No. Alcohol puts extra stress on an already damaged liver. Even small amounts can speed up scarring and increase the risk of cirrhosis. Doctors strongly recommend complete abstinence. This isn’t just about avoiding extra damage - it’s about giving your liver the best chance to heal and stay stable on treatment.
Does autoimmune hepatitis affect life expectancy?
With early diagnosis and consistent treatment, yes - life expectancy can be nearly normal. Studies show 94% of treated patients survive 10 years or more. Without treatment, survival drops to 10% in the same timeframe. The key is sticking to your medication schedule, attending regular check-ups, and managing side effects. Many patients live into their 70s and 80s with no major liver complications.
What are the signs that treatment isn’t working?
If your liver enzymes (ALT, AST) start rising again after being normal, that’s the first red flag. So is a return of symptoms - worsening fatigue, joint pain, or dark urine. Rising IgG levels also suggest inflammation is flaring up. If you notice any of these, contact your doctor immediately. Relapse is common if medication is stopped too soon or if doses are missed. Your treatment plan may need adjusting - maybe a higher dose, switching drugs, or adding a second-line agent like mycophenolate.
9 Comments
Nikhil Purohit
November 22, 2025 at 20:26 PM
Man, this post was super helpful - I’ve been dealing with crazy fatigue for months and my doctor just shrugged. Now I’m going back with this info and demanding the full antibody panel. Seriously, if you’re tired all the time and your liver enzymes are up? Don’t accept ‘it’s stress’ or ‘you’re just out of shape.’ This is real. I’m printing this out and showing my GP tomorrow.
Also, thanks for mentioning the bone health stuff - I didn’t realize steroids could wreck your bones. Gonna start taking calcium and lifting weights now.
Debanjan Banerjee
November 23, 2025 at 18:21 PM
While the clinical overview is accurate, there is a critical omission: the role of gut microbiome dysbiosis in triggering or exacerbating autoimmune hepatitis. Recent studies (e.g., Nature Gastroenterology, 2023) demonstrate that patients with AIH exhibit significant reductions in Faecalibacterium prausnitzii and increased Enterobacteriaceae abundance - both correlated with elevated ALT and IgG levels. Probiotic interventions, particularly Lactobacillus reuteri and Bifidobacterium longum, have shown promise in adjunctive therapy by modulating T-regulatory cell activity.
Additionally, the 2022 scoring system’s inclusion of anti-SLA/LP antibodies is a major advancement, but its sensitivity drops to 82% in non-Caucasian populations. Genetic heterogeneity - particularly in South Asian cohorts - necessitates population-specific validation. This is not just a Western disease; it’s underdiagnosed in India due to lack of access to specialized serology and biopsy infrastructure.
For patients on azathioprine, TPMT genotyping is essential. 10% of South Asians carry TPMT*3C alleles, rendering them hyper-sensitive to standard doses. Without testing, you risk life-threatening myelosuppression. This is not optional - it’s standard of care. Please advocate for this testing if you’re in a resource-limited setting.
Michael Marrale
November 25, 2025 at 09:41 AM
Hey, I know this sounds crazy but… have you ever heard of the ‘Liver Control Initiative’? I read this one forum post from a guy who said his doctor gave him steroids but then kept his bloodwork secret. He said his liver enzymes went down… but his phone started glitching. Like, weird text messages from unknown numbers about ‘immune calibration.’
And why do all the clinical trials happen in the US? What if the real cure is already out there - like in some Himalayan herb or something? They’re hiding it because pills make more money. I’m not saying I know for sure… but I’ve seen things. 🤫
David vaughan
November 27, 2025 at 07:07 AM
I’ve been on prednisone for 4 years… and I’m still here. 😔
My ALT was 680. Now it’s 28. I lost 40 lbs, got insomnia, and my bones feel like they’re made of chalk. I cry sometimes. Not because I’m weak - because I’m tired of being a statistic.
But I’m alive. And I’m not giving up. If you’re reading this and you’re scared? You’re not alone. I’m right here with you. 💙
Cooper Long
November 27, 2025 at 21:07 PM
The clinical accuracy of this post is commendable. However, the assertion that autoimmune hepatitis is 'not caused by lifestyle' warrants nuance. While not directly attributable to dietary or behavioral factors, emerging evidence suggests environmental triggers - including exposure to halogenated hydrocarbons, certain antibiotics, and even air pollution - may act as epigenetic catalysts in genetically predisposed individuals.
Furthermore, the term 'cured' is misleading. Remission is not eradication. The immune memory persists. The disease is dormant, not defeated. This distinction is critical for patient counseling and long-term management expectations.
Finally, the projection of a $2.04 billion market by 2028 is not merely a reflection of medical progress - it is a testament to the commodification of chronic autoimmune conditions in capitalist healthcare systems.
Sheldon Bazinga
November 29, 2025 at 05:54 AM
Bro… autoimmune hepatitis? More like Auto-Income-Hepatitis. They made this up so people would buy meds forever. I’ve been reading this stuff for 3 years now and I’m still alive. I don’t take any pills. Just drank lemon water and did yoga. My liver enzymes? Normal. Coincidence? Nah. They just don’t want you to know the truth.
Also, why do all the doctors look like they work for Pfizer? 🤔
Also also - if you’re not vegan, you’re part of the problem. Meat = inflammation = liver murder.
Sandi Moon
December 1, 2025 at 01:11 AM
How profoundly predictable. The Western medical establishment, armed with its sterile biopsies and FDA-approved regimens, presumes to have unlocked the mystery of a condition that has likely existed since the dawn of industrialized society. And yet, no one dares to ask: Why now? Why here? Why predominantly among white, middle-class women?
Perhaps it is not merely genetic susceptibility, but the cumulative toll of modernity: endocrine disruptors in our water, synthetic food additives, the psychological strain of late capitalism - all of which our immune systems, evolved for the Pleistocene, cannot possibly comprehend.
And yet, the solution remains the same: more steroids. More immunosuppressants. More compliance. More silence. The irony is not lost on those who have studied the history of medicine - we treat symptoms while ignoring causes, and then patent the cure.
Do not mistake control for healing.
Kartik Singhal
December 2, 2025 at 23:21 PM
Lmao this is so basic. Like, I read this and I’m like ‘wow, who wrote this? My 8th grade bio teacher?’ 😴
They didn’t even mention that AIH is 100% linked to glyphosate exposure. The WHO buried it. The FDA is in on it. My cousin’s neighbor’s dog got diagnosed with it after the farm sprayed Roundup next door. Coincidence? Nah. 🤡
Also, azathioprine? That’s just a fancy word for ‘I hate your liver.’
And why are all the studies from the US? Because they’re the ones selling the drugs. Go to India. Go to Africa. No one there has this ‘disease’ - because they don’t eat processed food. 🌱
Also, I’m not taking steroids. I’m taking turmeric. And I’m winning. 🙌




Julia Strothers
November 21, 2025 at 04:33 AM
So let me get this straight - the government and Big Pharma are using autoimmune hepatitis as a cover to pump steroids into people’s bodies so they can track them via their liver enzymes? 😏 I’ve seen the patents. Prednisone? It’s just a delivery system for microchips. And don’t get me started on azathioprine - that’s just a fancy name for slow-acting poison designed to make you dependent. They want you to believe it’s ‘immune system gone rogue’… but it’s not rogue. It’s REBELLING. And they’re scared of what happens when people wake up.
Also - why is the ‘Revised International Scoring System’ always updated right before a new drug patent drops? Coincidence? I think not.