Ledipasvir DILI Risk Assessment Tool
Personal Risk Assessment
Answer these questions based on your medical condition to calculate your risk of drug-induced liver injury (DILI) while taking ledipasvir.
Quick Takeaways
- Ledipasvir, a key part of modern hepatitis C therapy, has a low but real potential to cause drug‑induced liver injury (DILI).
- Most cases are mild, showing temporary rises in ALT/AST; severe injury is rare and usually linked to pre‑existing liver disease.
- Monitoring liver enzymes before and during treatment cuts the risk dramatically.
- Patients on other direct‑acting antivirals (DAAs) such as glecaprevir/pibrentasvir show similar safety profiles, but each drug has its own nuance.
- Talk to your prescriber if you notice jaundice, dark urine, or persistent fatigue while on therapy.
What Is Ledipasvir?
When treating hepatitis C, Ledipasvir is a direct‑acting antiviral that blocks the NS5A protein, halting viral replication. It was approved by the FDA in 2014 and is most commonly prescribed as the fixed‑dose combination Harvoni (ledipasvir‑sofosbuvir). The drug’s high cure rates-over 95 % for genotype 1-have made it a cornerstone of therapy worldwide.
Understanding Drug‑Induced Liver Injury (DILI)
Drug‑induced liver injury = a spectrum of liver damage caused by medications, supplements, or herbal products. It can range from harmless, transient enzyme elevations to fulminant liver failure. The liver’s role in metabolising compounds makes it vulnerable, especially when a drug is processed by the cytochrome P450 system or when the patient already has chronic liver disease.
How Ledipasvir Could Harm the Liver
Ledipasvir is eliminated mainly through the bile, with only a small fraction excreted unchanged in urine. While its metabolism bypasses the major CYP enzymes, it still interacts with transporters like P‑gp and BCRP. In rare instances, these interactions may disturb bile flow, leading to a buildup of bilirubin or enzymes such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Most DILI signals for ledipasvir appear as modest (1‑3× upper‑limit normal) ALT/AST spikes, often without symptoms. Severe hepatotoxicity-defined by ALT > 5× ULN plus bilirubin rise-is exceedingly uncommon, reported in <1 % of treatment courses.
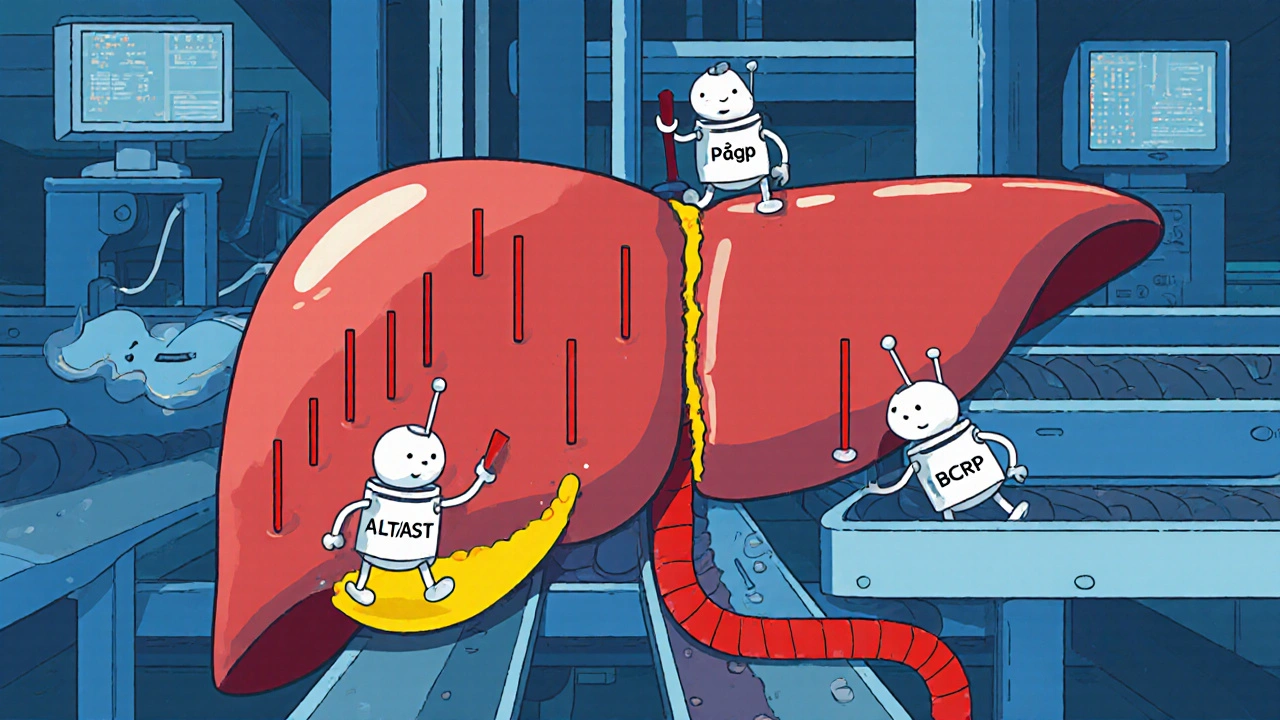
Clinical Evidence: What Trials and Real‑World Data Show
Phase III trials (ION‑1, ION‑3, ION‑4) enrolled > 2,000 participants. Across these studies, clinically significant liver injury occurred in only 5 participants (0.25 %). Most were patients with pre‑existing cirrhosis or co‑administration of hepatotoxic substances.
Post‑marketing surveillance up to 2024 adds another 30 confirmed DILI cases worldwide, reinforcing the rarity. A 2023 meta‑analysis of 12 cohort studies (n ≈ 9,800) found an average ALT elevation of 28 U/L (SD = 12) during treatment, returning to baseline within four weeks of therapy completion.
Who Is Most at Risk?
Identifying risk factors helps clinicians decide when extra monitoring is needed. The main predictors are:
- Baseline cirrhosis or advanced fibrosis (METAVIR F3‑F4).
- Concurrent use of other hepatotoxic drugs (e.g., certain anticonvulsants, statins at high dose).
- Alcohol misuse or heavy drinking (> 30 g/day for men, > 20 g/day for women).
- Genetic polymorphisms affecting bile‑acid transport (rare, but documented in case reports).
Patients without these factors typically experience only mild, self‑limiting enzyme changes.
Monitoring Guidelines: When and What to Test
Guidelines from the American Association for the Study of Liver Diseases (AASLD) recommend:
- Baseline liver panel (ALT, AST, bilirubin, alkaline phosphatase) before starting ledipasvir.
- Repeat testing at week 4 and week 8 of therapy.
- Additional testing if symptoms of jaundice, abdominal pain, or unexplained fatigue develop.
- Consider extending observation for 4‑6 weeks after treatment ends for patients with cirrhosis.
In most cases, a transient ALT rise < 3× ULN does not mandate stopping therapy; clinicians usually continue while monitoring weekly.
Comparison With Other Direct‑Acting Antivirals
| Drug (Combination) | Primary Metabolism Pathway | Reported Clinically Significant DILI | Special Monitoring Notes |
|---|---|---|---|
| Ledipasvir + Sofosbuvir | Bile excretion (Ledipasvir), nucleoside analogue (Sofosbuvir) | 0.25 % (severe) | Baseline & week 4/8 labs; extra care in cirrhosis |
| Glecaprevir + Pibrentasvir | CYP3A4 (Glecaprevir), minimal metabolism (Pibrentasvir) | 0.3 % (severe) | Watch for drug‑drug interactions; same lab schedule |
| Sofosbuvir + Velpatasvir | Nucleoside (Sofosbuvir), NS5A (Velpatasvir) | 0.2 % (severe) | Standard monitoring; low interaction risk |
As the table shows, ledipasvir’s liver‑injury profile sits comfortably within the range of other modern DAAs. The differences are subtle and largely driven by individual patient factors rather than intrinsic toxicity.
Practical Tips for Patients on Ledipasvir
- Keep a medication diary: note any over‑the‑counter drugs, herbal supplements, or alcohol.
- Schedule blood draws on the same day each week to track trends accurately.
- Stay hydrated; dehydration can falsely elevate liver enzymes.
- If you notice yellowing of the eyes or skin, dark urine, or persistent nausea, call your clinic immediately.
- Complete the full 8‑ or 12‑week course even if you feel better; stopping early raises the risk of resistance.
These simple habits help catch any red flags early and ensure the best chance of a cure without complications.
Future Directions: Is Ledipasvir Getting Safer?
Research continues to refine dosing and formulation. A 2025 phase‑II study explored a lower‑dose ledipasvir regimen for patients with mild fibrosis, showing comparable cure rates and even fewer ALT spikes. Meanwhile, pharmacogenomic screening for transporter polymorphisms is being piloted in a few European centers, aiming to personalize DILI risk assessment.
While we wait for these advances, the current data reassure that ledipasvir remains a safe, highly effective tool for most people battling hepatitis C.
Frequently Asked Questions
Can ledipasvir cause permanent liver damage?
Permanent damage is extremely rare. Most enzyme elevations resolve after treatment ends, especially when monitoring is followed.
What symptoms should make me stop the medication?
Severe jaundice, sharp right‑upper‑quadrant pain, or a sudden increase in bilirubin (> 2 mg/dL) warrant immediate medical review. Your doctor may pause or switch therapy.
Do I need to avoid alcohol while on ledipasvir?
Alcohol isn’t forbidden, but heavy drinking raises DILI risk. Moderation (≤ 14 units/week) is advised, and abstinence is best if you already have cirrhosis.
How often should my liver tests be done?
Baseline, then at weeks 4 and 8 of therapy. Additional checks are recommended if you develop any symptoms or have advanced liver disease.
Is it safe to take herbal supplements with ledipasvir?
Some herbs (e.g., St John’s wort) can affect liver transporters and raise DILI risk. Always discuss any supplement with your prescriber before starting treatment.
8 Comments
Mike Hamilton
October 18, 2025 at 20:26 PM
Well, i think its important to keep it simple. If you have a baseline lab and watch the trends you will feel a lot safer. The data shows most people dont have serious issues. It may not be perfect but its a good start.
Matthew Miller
October 19, 2025 at 00:03 AM
Yo, this stuff is like a rollercoaster of hope and caution! Ledipasvir knocks out the virus while the liver does a little dance on the sidelines. Most of the time it's just a tiny bump in the enzymes, but hey, that's why we keep tabs on them. Let’s celebrate the cure rate while we stay sharp on the labs!
Liberty Moneybomb
October 19, 2025 at 03:56 AM
The pharma giants are hiding the true danger of ledipasvir from the public!
Albert Fernàndez Chacón
October 19, 2025 at 07:50 AM
From a pharmacokinetic perspective, ledipasvir’s biliary excretion means we have to respect the liver’s transporters. If you’re already cirrhotic, those pathways are already strained, so monitoring becomes crucial. Even though the overall DILI rate is low, the stakes are higher when you add other hepatotoxic agents or alcohol. Keep your medication diary, stay hydrated, and let your clinician know about any over‑the‑counter supplements. Simple steps can keep the risk down.
Norman Adams
October 19, 2025 at 11:43 AM
Obviously the only person who actually reads the fine print is the one who thinks they’re above the average patient. Everyone else just nods and hopes for the best while the drug companies smile.
Margaret pope
October 19, 2025 at 15:36 PM
You can do this keep monitoring your labs and stay steady
Karla Johnson
October 19, 2025 at 19:30 PM
Let me lay it out clearly: first, the incidence of clinically significant DILI with ledipasvir is less than three in a thousand, which is statistically low, but it is not zero. Second, the risk factors-pre‑existing cirrhosis, concurrent hepatotoxic drugs, heavy alcohol use, and rare transporter polymorphisms-are well documented and should guide a personalized monitoring schedule. Third, baseline labs are non‑negotiable; you need ALT, AST, bilirubin, and alkaline phosphatase before you even consider starting therapy. Fourth, repeat labs at weeks four and eight provide a window into the drug’s hepatic impact, catching any upward trends before they become dangerous. Fifth, if you notice any symptom-jaundice, dark urine, unexplained fatigue-don’t wait for the next scheduled draw; contact your provider immediately. Sixth, the majority of enzyme elevations are modest, staying under three times the upper limit of normal, and these typically resolve spontaneously after treatment ends. Seventh, in the rare case of severe elevation-ALT >5× ULN with a bilirubin rise-clinical guidelines advise pausing therapy and reassessing the risk‑benefit ratio. Eighth, patient education is critical; a medication diary can prevent hidden interactions with over‑the‑counter products or herbal supplements that might tip the balance. Ninth, the role of alcohol cannot be overstated; even moderate consumption can amplify hepatic stress, especially in cirrhotic patients. Tenth, emerging pharmacogenomic screens for bile‑acid transporter variants hold promise for future risk stratification, but they are not yet standard practice. Eleventh, the recent lower‑dose studies suggest that dose adjustment might become a viable strategy for low‑risk patients, but until guidelines catch up, stick to the approved regimen. Twelfth, always complete the full course of therapy; early discontinuation raises the chance of viral resistance and defeats the purpose of a cure. Thirteenth, remember that the therapeutic benefit-over 95% cure rates for genotype 1-far outweighs the modest risk when proper monitoring is in place. Finally, keep a line of communication open with your healthcare team, because proactive management turns a potential problem into a manageable side effect.

Drew Waggoner
October 18, 2025 at 17:06 PM
I get upset when people act like liver enzymes are just a number that can be ignored. The fear of drug‑induced injury is real for many patients. Even mild ALT spikes can cause anxiety and affect quality of life. Staying informed and monitored is the only sane path forward.