Narrow therapeutic index drugs aren’t like other medications. A tiny change in dosage-maybe just 5% more or less-can mean the difference between effective treatment and dangerous side effects. These drugs demand precision. That’s why regulators around the world have set up stricter rules for generic versions of them. If you or someone you know takes warfarin, levothyroxine, or digoxin, understanding these rules isn’t just technical-it’s life-saving.
What Makes a Drug Have a Narrow Therapeutic Index?
A drug has a narrow therapeutic index (NTI) when the gap between a safe dose and a toxic one is very small. The FDA defines NTI drugs as those with a therapeutic index of 3 or less. That means the dose that causes harm is only three times higher than the dose that works. For comparison, most drugs have a therapeutic index of 10 or more.
Think of it like walking a tightrope. One misstep, and you fall. For patients on NTI drugs, even a small shift in how the body absorbs or processes the drug can lead to serious consequences. Too little warfarin? Risk of blood clots. Too much? Risk of internal bleeding. Too little levothyroxine? Fatigue, weight gain, heart problems. Too much? Heart palpitations, bone loss, even heart attack.
These aren’t hypothetical risks. In the 1970s and 80s, doctors started noticing that patients switched from brand-name to generic versions of drugs like phenytoin and digoxin sometimes had sudden changes in their blood levels. That led to therapeutic drug monitoring-regular blood tests to make sure levels stayed in the safe zone. But monitoring isn’t perfect. It’s expensive, inconvenient, and sometimes too late to prevent harm.
Why Generic Versions of NTI Drugs Need Extra Scrutiny
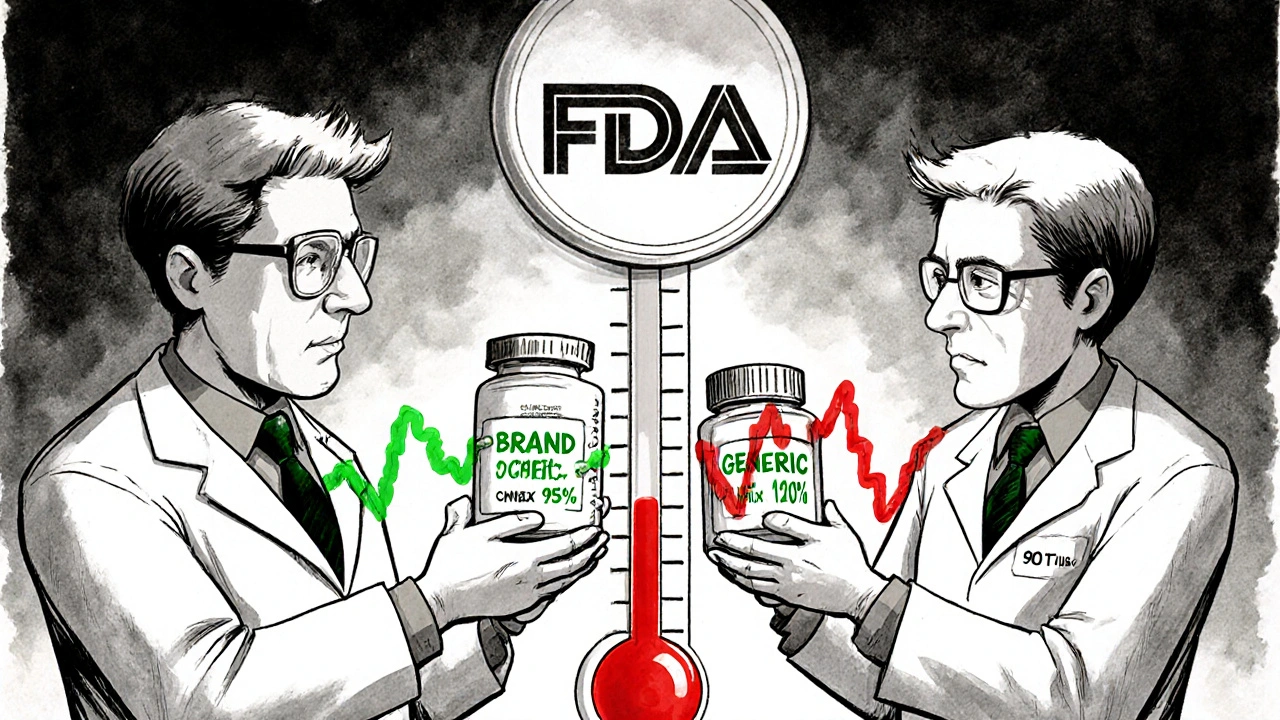
Generic drugs are supposed to be identical to their brand-name counterparts in active ingredient, strength, and performance. For most drugs, regulators accept bioequivalence if the generic’s blood concentration falls within 80% to 125% of the brand’s. That’s called the 80-125% rule.
But for NTI drugs, that range is too wide. A 125% increase in exposure might push a patient into toxicity. A 80% drop might make the drug useless. So regulators tightened the rules.
Here’s how different agencies handle it:
- U.S. FDA: Uses a three-part test: Reference-scaled average bioequivalence (RSABE), a variability comparison (test drug can’t be more variable than the brand), and unscaled average bioequivalence (still within 80-125%).
- European Medicines Agency (EMA): Uses a fixed range of 90-111% for both AUC and Cmax.
- Health Canada: Requires 90.0-112.0% for AUC in critical dose drugs.
The FDA’s approach is the most complex. It doesn’t just look at average exposure-it checks how consistent the drug is from dose to dose in the same person. If the generic varies more than the brand, it fails-even if the average is perfect. This matters because some patients are extra sensitive to fluctuations.
How Bioequivalence Studies for NTI Drugs Are Different
Testing a generic NTI drug isn’t like testing a regular one. Standard bioequivalence studies use 24-36 healthy volunteers. For NTI drugs, studies need 36 to 54 participants. Why? Because you need more data to detect small differences in variability and exposure.
The design is more demanding too. The FDA often requires a fully replicated crossover design-meaning each participant takes both the brand and generic multiple times, in different orders. This lets researchers measure within-subject variability, which is critical for NTI drugs.
These studies cost more. A standard generic bioequivalence study might run $300,000-$700,000. For NTI drugs, the cost jumps to $500,000-$1 million. That’s a big barrier for smaller generic manufacturers. It’s one reason why fewer NTI generics are on the market.
Which Drugs Are Classified as NTI?
The FDA doesn’t publish a single official list of NTI drugs. Instead, it issues product-specific guidance for each one. As of 2023, there are about 35 drugs globally recognized as NTI. The most common ones include:
- Warfarin
- Levothyroxine
- Digoxin
- Phenytoin
- Carbamazepine
- Tacrolimus
- Sirolimus
- Lithium carbonate
- Theophylline
- Valproic acid
These drugs are used for conditions that require precise control: heart rhythm disorders, thyroid replacement, epilepsy, organ transplant rejection, and mood stabilization. Many are taken long-term, sometimes for life. That means even small differences in how a generic performs can add up over months or years.
Real-World Evidence Shows Generic NTI Drugs Can Be Safe
Despite the concerns, real-world data supports the safety of generic NTI drugs when they meet the stricter bioequivalence standards.
A 2017 study in the American Journal of Transplantation looked at kidney transplant patients switched from brand-name tacrolimus to a generic version that passed FDA’s NTI criteria. There was no increase in rejection rates or toxicity. Another study in Circulation: Cardiovascular Quality and Outcomes in 2019 found no difference in bleeding or clotting events between patients taking brand or generic warfarin.
These findings matter. They show that when the science is followed-when generics are tested under the right conditions-they work just as well.

Why the Debate Still Exists
Not everyone agrees on how strict the rules should be. Some experts, like Dr. Leslie Benet from UCSF, say the FDA’s approach is scientifically sound. It protects patients without being arbitrary.
Others, like Dr. Lawrence Lesko, worry the rules are too strict. He argues that for some NTI drugs with very low variability-like levothyroxine-the extra testing adds cost without adding safety. He fears it could reduce competition, keeping prices high and limiting patient access.
There’s also the issue of confusion. Because the FDA doesn’t have a single list, pharmacists and doctors don’t always know which drugs are classified as NTI. That leads to unnecessary caution. Some prescribers refuse to switch patients to generics even when the drug has passed all regulatory tests.
What’s Changing in 2024 and Beyond
The FDA is working on a more systematic way to classify NTI drugs. Right now, it’s case-by-case. In 2022, the agency released a pharmacometric approach using quantitative thresholds to identify NTI drugs. By mid-2024, they plan to issue final guidance on RSABE for NTI drugs, making the rules clearer and more consistent.
There’s also talk of global harmonization. The EMA, Health Canada, and FDA are talking about aligning their standards. If they can agree on a common framework, it could cut development costs by 15-20%. That might encourage more generic manufacturers to enter the market.
For patients, that could mean more affordable options. For the system, it could mean fewer costly hospitalizations from drug toxicity or treatment failure.
What This Means for Patients
If you’re on an NTI drug, you don’t need to panic about generics. But you should be informed.
- Ask your doctor or pharmacist: Is this a narrow therapeutic index drug?
- If you’re switched to a generic, monitor for new symptoms-fatigue, dizziness, irregular heartbeat, unusual bruising.
- Don’t assume all generics are the same. If you’ve had issues with one generic, talk to your provider before switching again.
- Keep your blood tests on schedule. Even with strict bioequivalence rules, monitoring is still part of safe care.
The goal isn’t to scare you away from generics. It’s to make sure the system works so you can trust them. The science is there. The rules are tight for a reason. And when followed correctly, generic NTI drugs are safe, effective, and life-changing for millions.
Are all generic drugs held to the same bioequivalence standards?
No. Most generic drugs must show bioequivalence within 80-125% of the brand-name drug. But for narrow therapeutic index (NTI) drugs, the standards are much tighter. The FDA requires additional criteria, including reference-scaled bioequivalence and a variability comparison. The EMA and Health Canada use fixed, narrower ranges like 90-111% or 90-112%.
Why are NTI drugs more dangerous if the generic isn’t perfectly matched?
Because the difference between a therapeutic dose and a toxic dose is very small. For example, with warfarin, a 10% increase in blood concentration can raise bleeding risk significantly. With levothyroxine, even a 5% change can affect heart rate, weight, and mood. These drugs don’t have a safety buffer-so small variations in absorption or metabolism can have big consequences.
Can I trust generic versions of my NTI drug?
Yes-if the generic has passed the stricter bioequivalence requirements set by your country’s regulator. Studies have shown that generics of drugs like tacrolimus and warfarin, tested under FDA’s NTI guidelines, perform just as safely and effectively as the brand. The key is ensuring the generic was tested under the correct, tighter standards-not just the standard 80-125% rule.
What’s the difference between the FDA and EMA approaches to NTI drugs?
The FDA uses a flexible, science-based method called reference-scaled average bioequivalence (RSABE), which adjusts limits based on how variable the brand drug is. It also checks whether the generic is more variable than the brand. The EMA uses a fixed, tighter range (90-111%) regardless of variability. The FDA’s method is more complex and costly but can be more precise for drugs with moderate variability.
Why aren’t there more generic NTI drugs on the market?
Because the testing requirements are expensive and complex. Bioequivalence studies for NTI drugs cost $500,000-$1 million, compared to $300,000-$700,000 for regular generics. Many manufacturers avoid them due to high costs and regulatory uncertainty. Also, the FDA doesn’t have a clear, public list of NTI drugs, making it harder for companies to know which ones require special testing.
Should I avoid switching from brand to generic for NTI drugs?
Not necessarily. Many patients successfully switch without issues. But because NTI drugs require precision, it’s important to talk to your doctor before switching. Monitor for any new symptoms after the switch, and keep up with blood tests. If you’ve had problems with one generic, don’t assume another will be the same-some generics may pass regulatory standards but still affect you differently due to inactive ingredients or formulation differences.
11 Comments
Ryan Airey
November 15, 2025 at 22:25 PM
Let’s be real-the FDA’s RSABE nonsense is just corporate protectionism dressed up as 'science.' If a generic passes 80-125%, it’s fine. The rest is just a money grab by brand-name pharma to keep prices high. Patients are paying the price for overregulation.
Hollis Hollywood
November 16, 2025 at 03:27 AM
I get why people are nervous. I had a cousin who went into atrial fibrillation after switching generics for levothyroxine. Turned out it was a bad batch-different filler, not the active ingredient. But that’s the thing: even when the science says it’s safe, the human experience doesn’t always line up. We need better tracking, not just stricter tests. Maybe a national database for generic switches and adverse events? Could save lives.
Aidan McCord-Amasis
November 16, 2025 at 09:12 AM
Generic NTI drugs = 🤡
My grandma’s digoxin levels went nuts after switching. Now she’s in rehab. 💀
Adam Dille
November 16, 2025 at 14:41 PM
Honestly, I think we’re overcomplicating this. If the generic meets the tighter standards, it’s safe. If it doesn’t, it shouldn’t be sold. But we need to make sure pharmacists and doctors actually know which drugs are NTI. I’ve seen people get switched to generics for tacrolimus without even knowing what they’re on. That’s the real problem-not the science, it’s the communication.
Katie Baker
November 17, 2025 at 01:16 AM
My dad’s on lithium and switched generics last year. He was scared to death, but we did it together-tracked his mood, his sleep, his tremors. Nothing changed. Honestly? I’m so glad we did it. Generic saved us like $200 a month. We’re not scared anymore. 💪
John Foster
November 17, 2025 at 18:32 PM
There’s a deeper truth here, buried beneath the regulatory jargon and pharmacokinetic curves. We live in an age where we outsource our bodily autonomy to algorithms and FDA guidelines. We trust that a number-90-111%-can replace the sacred, intimate relationship between patient and medicine. But what is a drug, really? Is it the molecule? Or is it the ritual-the daily pill, the blood draw, the silent prayer that today, today, the body will not betray us? The generic is not a substitute. It is a mirror. And some of us, when we look into it, see only the shadow of our own fragility.
Edward Ward
November 18, 2025 at 16:28 PM
Wait-so the EMA uses a fixed 90-111% range, but the FDA uses RSABE which adjusts based on variability? That’s actually brilliant. It means the FDA is adapting to the drug’s behavior, not forcing every drug into the same box. But why isn’t this more widely known? I’ve seen pharmacists refuse to fill generics for warfarin because they think 'all generics are the same.' That’s dangerous. We need better education-not just tighter rules. And why isn’t there a public, searchable database of NTI drugs? It’s 2024. We have apps for everything. Why not this?
Andrew Eppich
November 19, 2025 at 18:28 PM
It is both a moral and scientific imperative that the standards for drugs with narrow therapeutic indices be maintained at the highest level of rigor. To relax these standards is to invite catastrophe. The patient’s life is not a variable to be optimized for cost-efficiency. The integrity of medical practice demands nothing less than precision.
Jessica Chambers
November 21, 2025 at 09:45 AM
Oh, so now the FDA is the hero? 😏
Meanwhile, my pharmacy switched me to a generic tacrolimus that cost $12 instead of $400… and my creatinine spiked. Guess what? They didn’t even tell me it was an NTI drug. Thanks, system.
Shyamal Spadoni
November 21, 2025 at 15:57 PM
you think this is about safety? lol. big pharma and the fda are in bed together. the real reason they make it so hard to make ntis generic is so they can keep charging $1000/month for warfarin. and dont get me started on how they use 'variability' as an excuse. its all smoke and mirrors. i read a paper once (somewhere on the dark web) that said the 80-125% rule is actually safer because it allows for natural variation in patients. they just want to control the market. and dont even get me started on the filler chemicals in generics-i think they’re laced with microchips. i know it sounds crazy but my cousin’s cat got weird after eating a generic pill. just saying.




ASHISH TURAN
November 15, 2025 at 13:22 PM
Been on warfarin for 8 years. Switched to generic last year after my insurance forced it. No issues. Bloodwork stayed stable. Doctors always say 'trust the science'-turns out they’re right. Just keep your INR checks regular, and you’ll be fine.