Tag: bioequivalence
Clinical Studies on Generic Drug Efficacy: What the Research Really Shows
Clinical studies show generic drugs are just as effective as brand-name versions for most people. Learn what the research says about bioequivalence, rare exceptions, and when to stick with one brand.
View More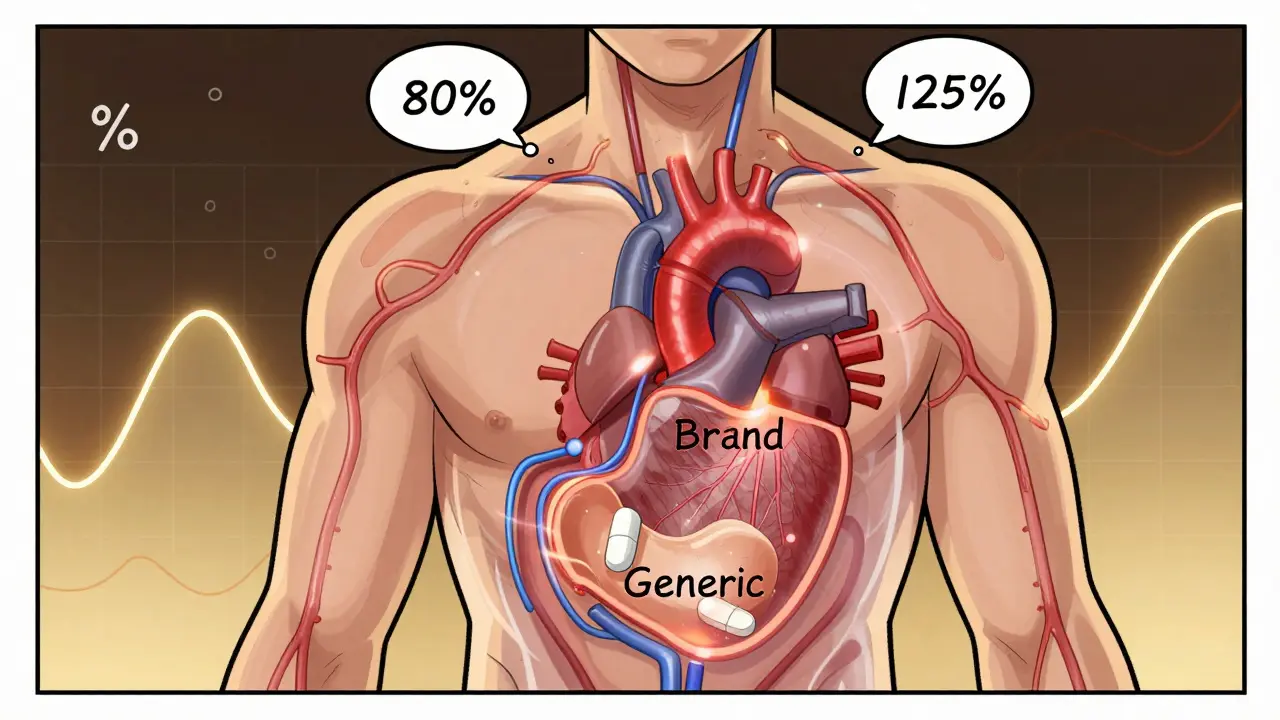
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals in Generic Drugs
The 80-125% rule ensures generic drugs behave like brand-name versions in the body. It's based on pharmacokinetic data, not pill content. Here's how it works and why it matters.
View More