Generic Drugs Safety: What You Need to Know Before Taking Them
When you hear generic drugs, medications that contain the same active ingredients as brand-name drugs but cost far less. Also known as generic medications, they are approved by the FDA, the U.S. agency that ensures drugs are safe, effective, and properly labeled to work just like the original. For over 90% of prescriptions in the U.S., generics are the default choice—not because they’re cheaper, but because they’re proven to be just as effective. But safety isn’t just about whether the pill works. It’s about where it comes from, how it’s made, and whether you can trust the source.
Not all generic drugs are created equal, even if they carry the same active ingredient. The FDA approval, the process that verifies a generic drug matches the brand-name version in strength, dosage, and how it’s absorbed by the body is strict—but it only applies to drugs sold legally in the U.S. If you’re buying from an unverified online pharmacy, you could get a pill with the wrong dose, no active ingredient, or even toxic fillers. Real-world cases have shown counterfeit versions of common drugs like metoprolol, warfarin, and tetracycline showing up online, often disguised as legitimate generics. The FDA doesn’t regulate foreign websites, so if a deal seems too good to be true, it probably is.
Even within legal channels, differences in inactive ingredients can matter. One person might react to a dye or filler in a generic version of lorazepam that they never noticed with the brand name. That’s why health literacy matters—reading labels, knowing your pharmacist, and asking about changes in how a pill looks or tastes can prevent problems. Studies show that patients who understand their medication labels are less likely to make dosing mistakes, especially with drugs like anticoagulants or epilepsy meds where small errors can be dangerous.
Some generics are made in the same factories as brand-name drugs. Others are made overseas under different oversight. The drug equivalence, the scientific standard that proves a generic performs the same way in the body as the original is measured through bioequivalence tests—but those tests don’t catch every variation in manufacturing quality. That’s why sticking to well-known U.S. pharmacies, checking for FDA-registered facilities, and avoiding bulk buys from unknown sellers is your best defense.
You don’t need to pay more to get safe medication. But you do need to know where to look. The posts below cover real cases: how tamoxifen and warfarin generics are monitored, what happens when you mix generics with other drugs like antipsychotics or blood thinners, and how to spot fake pills sold as cheap generics. Whether you’re managing high blood pressure, epilepsy, or anxiety, the key isn’t avoiding generics—it’s choosing them wisely.
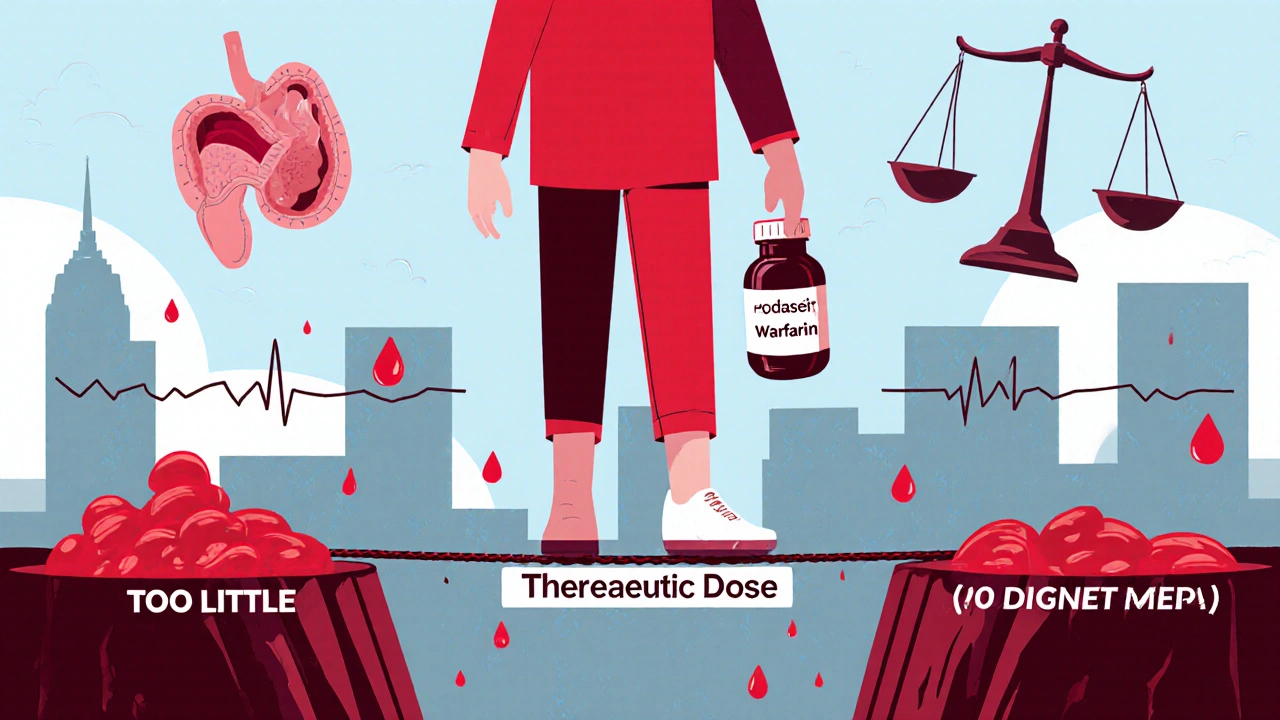
Narrow Therapeutic Index Drugs: Why Bioequivalence Standards Are Tighter for These Medications
Narrow therapeutic index drugs require stricter bioequivalence standards because small differences in dosage can cause serious harm. Learn how regulators like the FDA and EMA ensure generic versions are safe and effective.
View More