Category: Medications - Page 3

Understanding Medication Use During Pregnancy: Safety Alerts and What You Need to Know
Learn how pregnancy medication safety alerts work, what drugs are risky, and how to make informed choices. Understand the shift from letter ratings to real data, and what steps to take for your health and your baby's.
View More
Combination Drug Substitution: Legal and Practical Challenges Explained
Combination drug substitution sounds like a simple way to cut costs and simplify treatment, but legal restrictions and safety risks make it far more complex than it appears. Here’s what patients and pharmacists need to know.
View More
Copay Assistance for Generics: How to Find Financial Help for Prescription Medications
Find out how to get financial help for generic prescription drugs. Learn about pharmacy discounts, Medicare Extra Help, nonprofit aid, and the 2025 changes that could cut your costs by more than half.
View More
Cardiovascular Generics: What Safety Studies and Real-World Data Really Show
Cardiovascular generics save billions but raise safety questions. Real-world data shows they work for most people - but some patients experience more side effects after switching. Here’s what the studies really say.
View More
SSRIs and Anticoagulants: What You Need to Know About the Bleeding Risk
Combining SSRIs with anticoagulants increases bleeding risk by 33%, especially in the first 30 days. Learn why platelet effects matter, which drugs are riskiest, and what alternatives exist.
View More
Adverse Event Monitoring for Biosimilars: How Safety Surveillance Works in Real-World Use
Biosimilars offer cost savings but require specialized safety monitoring due to their complex nature. Learn how adverse event tracking works, why traceability matters, and what patients and providers can do to ensure safety.
View More
Medications with a Narrow Therapeutic Index: Why Expired Pills Can Be Dangerous
Expired medications with a narrow therapeutic index-like warfarin, lithium, and digoxin-can become dangerous even with small potency changes. Learn why these drugs demand strict expiration compliance and what to do if yours has expired.
View More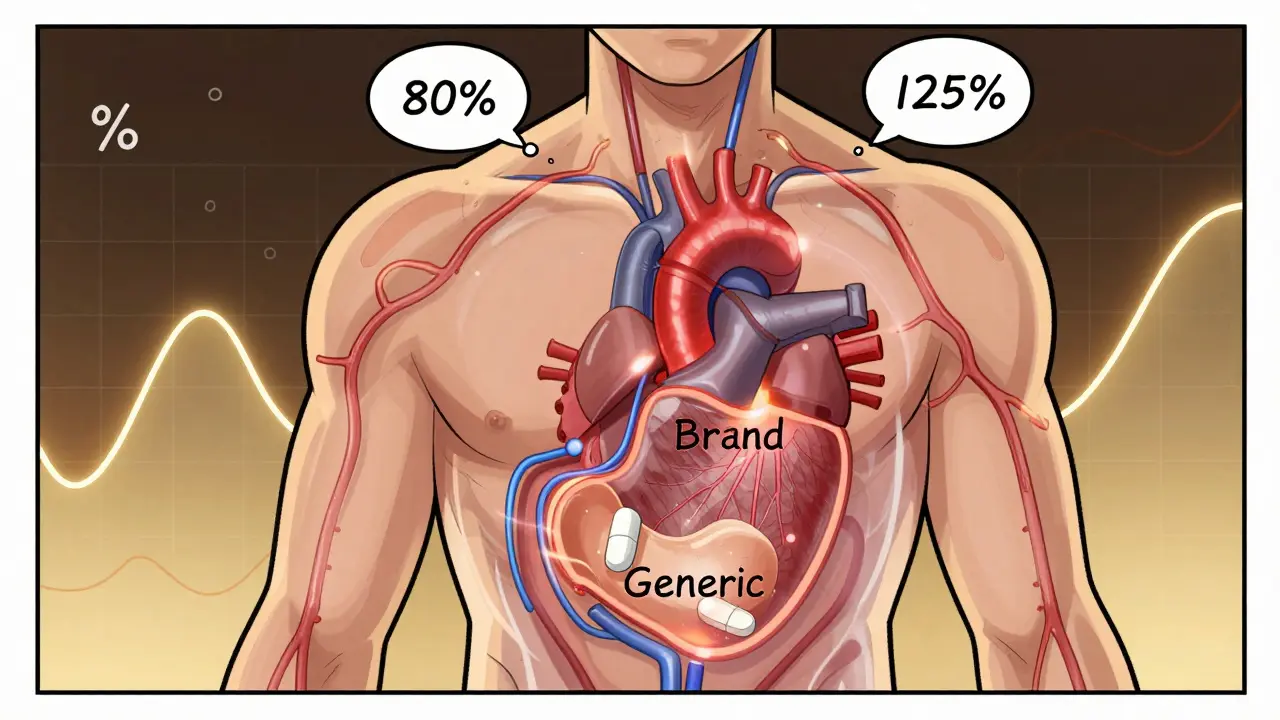
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals in Generic Drugs
The 80-125% rule ensures generic drugs behave like brand-name versions in the body. It's based on pharmacokinetic data, not pill content. Here's how it works and why it matters.
View More
Elderly Patients Switching to Generics: What You Need to Know About Safety, Cost, and Adherence
Switching elderly patients to generic medications can save money but carries risks if not handled carefully. Learn which drugs are safe to switch, when to monitor closely, and how to improve adherence with simple communication strategies.
View More
Child-Resistant Containers and Medication Safety Caps Explained: How They Work and Why They Matter
Child-resistant packaging has saved hundreds of thousands of children from accidental poisonings since 1970. Learn how these safety caps work, which medications require them, why they sometimes fail, and what you can do to keep kids safe.
View More